BACKGROUND: Immunosuppressive therapy (IST) (anti-thymocyte immunoglobulin (ATG) and cyclosporine (CSA)) is the treatment of choice for patients with acquired severe aplastic anemia (SAA) who are ineligible for hematopoietic stem cell transplantation (HSCT), which results in a hematological response (HR) in about 60-70% of SAA patients, while 1/3 of patients fail to achieve a hematological response. TPO is a hepatic-secreted hematopoietic growth factor that specifically regulates platelet production, induces hematopoietic stem cell differentiation to megakaryocytes by binding to the TPO receptor (MPL), stimulates megakaryocyte proliferation and differentiation, and promotes platelet production and release. Previous retrospective studies have demonstrated that IST combined with rhTPO improves the early hematologic response in SAA patients, but efficacy reported by centers varied depending on the dose and duration of rhTPO. There are no prospective studies of rhTPO application in SAA to confirm its efficacy. So this study was designed to observe the early feeicacy and safety of the SAA patients who were treated with IST combination with full-dose rhTPO.
METHODS: This study was a single-center, single-arm, prospective cohort clinical study, enrolling 33 naive SAA patients aged between 14-70 years who diagnosed after April 2021 in our study center. The patients treated with IST combined with full-dose rhTPO (300U/kg.d) for 8 weeks starting from the first day of ATG. The primary endpoint of the observational study protocol good response rate, blood product transfusion volume and safety profile at 3 months of treatment of initial SAA. The patients were also evaluated for total hematologic response rate and adverse effects of drug application, clonal shift rate, and recurrence rate at 3 and 6 months after treatment.
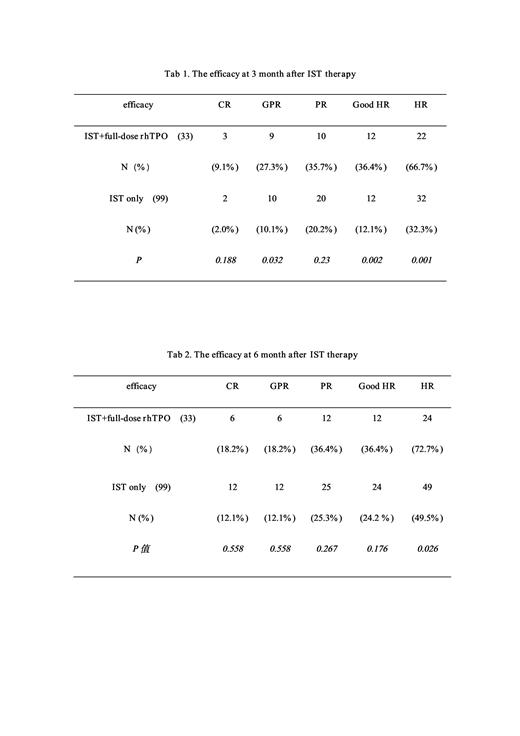
Efficacy Criteria:Complete response (CR): HGB>100 g/L, PLT> 100×10 9/L and ANC>1.5×10 9/L; ② Good partial response (GPR): HGB>80g/L, PLT> 50×10 9/L and ANC>1.0×10 9/L; ③ Partial response (PR): withdrawal from blood product transfusion dependence (HGB>70g/L, PLT> 20×10 9/L), hematologic tests improved and no longer met the SAA criteria, but blood count did not meet the GPR criteria. ④No response to treatment (NR): Continuous transfusion dependency was classified as no response. and/or blood count still met SAA criteria. We defined CR+GPR+PR as HR and CR+GPR as good HR.
RESULTS: The rhTPO group enrolled 33 patients, and the control group was 99 patients who applied the caliper value of 0.2 to match the previous unapplied TPO treatment of SAA. At 3 months after IST treatment efficacy, the HR rate of 33 patients in the rhTPO group was 66.67% (22 /33 ), and the rate of good HR was 36.4% (12/33). The HR rate in the control group was 32.32% (32 /99 ), and the rate of good HR was 12.12% (12/99). The HR rate and the good HR rate between the two group were significantly statistical differences (66.67% v 32.32%; 36.4% v 12.12%), (P value=0.001; 0.002). At 6 months after IST treatment, the HR rate and good HR rate in the rhTPO group had a significantly higher than those of the control group (66.67% v 32.32%; 36.4% v 12.12%), (P value=0.001; 0.002).
No disease transformation such as myelofibrosis, myelodysplastic syndrome and acute myeloid leukemia occurred in all enrolled patients during the follow-up period.
CONCLUSION: 22 of the 33 SAA patients in this study achieved HR at 3 months after IST treatment, including 36.4% cases achived good HR , which is the highest good efficacy response in a prospective study of SAA patients in China known to us to date. This confirms that full-dose rhTPO, as a macromolecular peptide, can also promote the recovery of bone marrow hematopoietic function, significantly improved the rapidity and strength of HR in SAA patients. And its clinical effect is no less than that of the various TPO-Ras that have been widely used so far, and may even be higher.
【Keywords】severe aplastic anemia; recombinant human thrombopoietin; immunosuppression treatment.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal